- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
What is the Process Involved in Electrodepositing Copper Using a Titanium Electrode?
Electrodeposition of copper using titanium electrodes is a fascinating process in the world of electrochemistry and materials science. This technique involves the use of an electric current to deposit copper onto a substrate, combining the excellent properties of both metals. The process has gained significant attention in various industries due to its ability to create high-quality deposition with enhanced durability and conductivity. In this blog post, we'll explore the intricacies of this electrodeposition process, its applications, and the key factors that influence its success.
How does the electrodeposition of copper work?
The electrodeposition of copper on substrate is a complex electrochemical process that involves several steps and carefully controlled conditions. To understand this process, we need to delve into the fundamentals of electrochemistry and the specific characteristics of both copper and titanium.

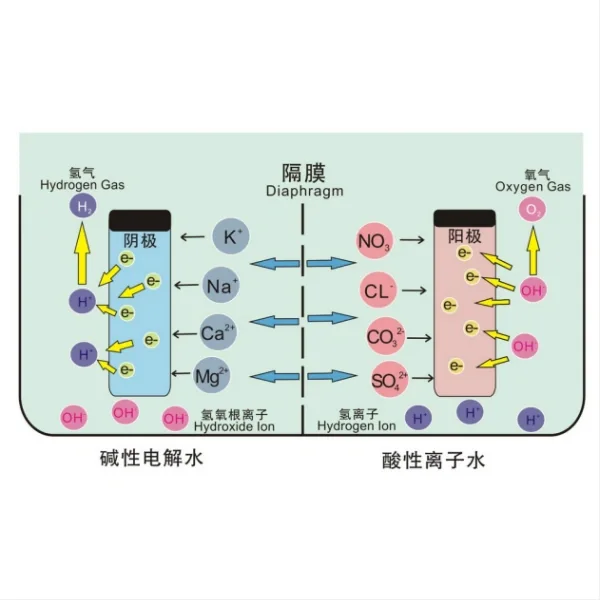
At its core, the electrodeposition process involves the reduction of copper ions from an electrolyte solution onto a cathode. The titanium electrode serves as the anode. This process takes place in an electrochemical cell, which typically consists of the following components:
1. Anode: Usually made of titanium anode coated with mixed metal oxide.
2. Cathode: The substrate which copper will be deposited.
3. Electrolyte: An aqueous solution containing copper ions, typically copper sulfate (CuSO4) dissolved in water, along with other additives to enhance conductivity and control the deposition process.
4. Power source: A direct current (DC) power supply that provides the necessary electrical energy for the reaction.
The electrodeposition process begins when an electric current is applied to the system. This causes the titanium anode to oxidize. Simultaneously, at the titanium cathode, copper ions from the electrolyte are reduced and deposited as metallic copper (Cu) onto the cathode.
Several factors influence the quality and characteristics of the deposited copper:
1. Current density: The amount of current applied per unit area of the electrode surface affects the rate of deposition and the properties of the resulting copper deposition.
2. Electrolyte composition: The concentration of copper ions, pH, and presence of additives in the electrolyte solution can significantly impact the deposition process and the properties of the deposited copper.
3. Temperature: The temperature of the electrolyte solution affects the rate of deposition and the morphology of the deposited copper.
4. Agitation: Stirring or flowing the electrolyte solution can help maintain a uniform concentration of copper ions near the cathode surface, leading to more even deposition.
5. Substrate preparation: The condition of the titanium surface, including its cleanliness and roughness, can affect the adhesion of mixed metal oxide.
6. Deposition time: The duration of the electrodeposition process determines the thickness of the copper deposition.
The mixed metal oxide coating on titanium offers several advantages. The resulting composite material combines the excellent corrosion resistance and high strength-to-weight ratio of titanium with the superior electrical and thermal conductivity of mixed metal oxide.
What are the key factors affecting the quality of copper electrodeposition?
The quality of copper deposition by titanium electrode is crucial for its performance in various applications. Several key factors influence the deposition process and the resulting copper's properties. Understanding and controlling these factors is essential for achieving high-quality deposition that meet specific requirements.

1. Current density:
The current density, which is the amount of electrical current flowing per unit area of the electrode surface, is one of the most critical parameters in electrodeposition. It directly affects the rate of copper deposition and the morphology of the deposited copper.
- Low current density: Generally results in slower deposition rates and can lead to a more compact and fine-grained copper structure. However, if the current density is too low, it may result in poor adhesion or incomplete coverage.
- High current density: Increases the deposition rate but can lead to rougher, less uniform deposits. Extremely high current densities may cause "burning" or dendritic growth of the copper, resulting in a powdery or spongy deposit.
The optimal current density depends on the specific application and desired properties of the copper deposition. It often requires experimentation and fine-tuning to achieve the best results.
2. Electrolyte composition:
The composition of the electrolyte solution plays a crucial role in the electrodeposition process. The main components of the electrolyte include:
- Copper salt: Typically copper sulfate (CuSO4), which provides the source of copper ions.
- Sulfuric acid: Added to increase conductivity and maintain the pH of the solution.
- Additives: Various organic and inorganic compounds that can be added to modify the properties of the deposited copper.
The concentration of copper ions in the electrolyte affects the deposition rate and the quality of the deposit. A higher concentration generally allows for higher current densities and faster deposition rates. However, it's essential to maintain the copper ion concentration within an optimal range to ensure consistent deposition quality.
Additives in the electrolyte can significantly influence the properties of the deposited copper. Common additives include:
- Leveling agents: Help to produce smoother, more uniform deposits by preferentially inhibiting deposition on protrusions.
- Brighteners: Promote the formation of smaller grain sizes, resulting in a brighter, more reflective copper surface.
- Stress reducers: Help to minimize internal stresses in the deposited copper layer, reducing the risk of cracking or peeling.
- Wetting agents: Improve the wetting of the titanium surface, enhancing adhesion and coverage.
3. pH of the electrolyte:
The pH of the electrolyte solution affects the efficiency of the deposition process and the properties of the deposited copper. For copper electrodeposition, the optimal pH range is typically between 0.5 and 2.0.
- Lower pH (more acidic): Tends to produce finer-grained deposits but may increase hydrogen evolution, which can lead to embrittlement of the deposit.
- Higher pH: Can result in coarser-grained deposits and may lead to the formation of basic copper salts, which can interfere with the deposition process.
Careful control of the pH is necessary to maintain consistent deposition quality and prevent unwanted side reactions.
4. Temperature:
The temperature of the electrolyte solution affects various aspects of the electrodeposition process:
- Deposition rate: Higher temperatures generally increase the deposition rate due to increased ion mobility and reaction kinetics.
- Grain size: Elevated temperatures often lead to larger grain sizes in the deposited copper.
- Stress: The internal stress in the deposited copper can be influenced by temperature, with higher temperatures typically resulting in lower stress.
- Additive effectiveness: The performance of certain additives in the electrolyte may be temperature-dependent.
The optimal temperature range for copper electrodeposition is typically between 20°C and 30°C, although this can vary depending on the specific application and desired properties.
5. Agitation:
Proper agitation of the electrolyte solution is crucial for achieving uniform and high-quality copper deposits. Agitation serves several purposes:
- Maintains a uniform concentration of copper ions near the cathode surface, preventing depletion.
- Helps to remove hydrogen bubbles that may form on the cathode surface, which can interfere with the deposition process.
- Promotes mass transfer of ions, improving the overall efficiency of the process.
Agitation can be achieved through various methods, including mechanical stirring, air sparging, or solution flow. The optimal level of agitation depends on factors such as the geometry of the electrodeposition setup, the current density, and the desired properties of the copper deposit.
By carefully controlling and optimizing these key factors, it's possible to achieve high-quality electrodeposited copper with the desired properties for specific applications. This may involve a process of experimentation and fine-tuning to determine the optimal parameters for a given set of requirements.
The versatility of titanium electrodes continues to drive innovation across these and other industries. As manufacturing techniques improve and new applications are discovered, we can expect to see even more diverse uses for these unique composite materials in the future.

In conclusion, the process of electrodepositing copper using a titanium electrode is a sophisticated technique that combines the beneficial properties of both metals. By carefully controlling the various factors involved in the electrodeposition process, it's possible to create high-quality coatings that meet the specific requirements of a wide range of applications. From electronics and energy storage to aerospace and medical devices, copper-electrodeposited titanium electrodes continue to play a crucial role in advancing technology across numerous industries. As research in this field progresses, we can anticipate even more innovative applications and improved performance of these versatile materials in the future.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Walsh, F. C., & Ponce de León, C. (2014). A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: an established and diversifying technology. Transactions of the IMF, 92(2), 83-98.
2. Schlesinger, M., & Paunovic, M. (Eds.). (2011). Modern electroplating (Vol. 55). John Wiley & Sons.
3. Low, C. T. J., Wills, R. G. A., & Walsh, F. C. (2006). Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surface and Coatings Technology, 201(1-2), 371-383.
4. Datta, M., & Landolt, D. (2000). Fundamental aspects and applications of electrochemical microfabrication. Electrochimica Acta, 45(15-16), 2535-2558.
5. Fujimoto, S., Hayashi, S., & Nakao, T. (2001). Improvement of pitting corrosion resistance of titanium by copper electrodeposition. Materials Science and Engineering: A, 308(1-2), 268-273.
6. Eliaz, N., & Gileadi, E. (2008). Physical electrochemistry: fundamentals, techniques, and applications. John Wiley & Sons.
7. Paunovic, M., Schlesinger, M., & Snyder, D. D. (2010). Fundamental considerations. Modern Electroplating, 1-32.
Related Industry Knowledge
- How Do Titanium Electrodes Enhance Copper Electrodeposition Efficiency and Quality?
- How to Prepare a Titanium Electrode for Copper Electrodeposition?
- Can Copper Be Used as an Electrode?
- Are There Environmental Benefits to Using Titanium Anodes for Copper Electrowinning?
- Can Titanium Electrodes Improve the Adhesion of Copper Electrodeposition?
- Why Should You Consider Titanium Electrodes for Copper Plating?
- What Makes PCB RPP Copper Plating Essential for Durable Surface Area Enhancement?
- How Does PCB VCP DC Copper Plating Work in Direct Current Systems?